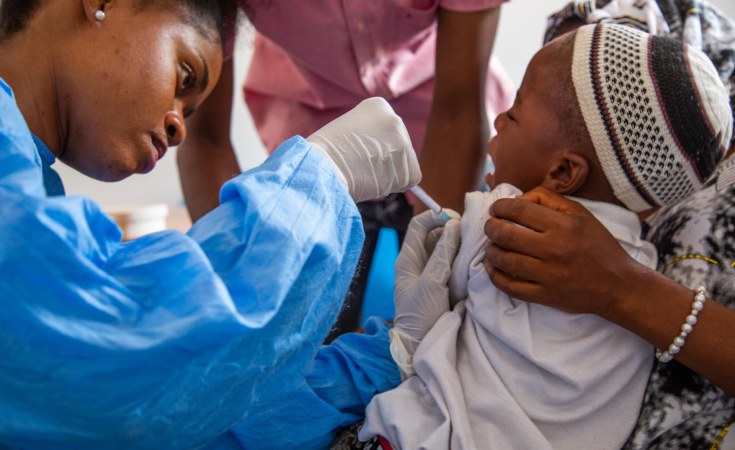
London/Douala/Lagos — Vaccine developers must share the know-how behind a groundbreaking new malaria jab so it can be rolled out efficiently to poor populations in Africa that need it most, according to a senior malaria advisor to the continent's main healthcare agency.
"We need to start having this conversation around tech transfer and IP," said Nicaise Ndembi, advisor to the director general of Africa CDC, based in Addis Ababa, Ethiopia.
"All these pharmaceutical companies are not based in Africa and the problem is in Africa."
In 2021, 619,000 people died from malaria, 96 per cent of them in the Africa region, according to WHO figures.
"All these pharmaceutical companies are not based in Africa and the problem is in Africa."Nicaise Ndembi, advisor to the director general, Africa CDC
The R21/Matrix-M vaccine, developed by the University of Oxford and the Serum Institute of India, is the second malaria vaccine to be recommended by the WHO, after the RTS,S vaccine (Mosquirix) was approved in 2021.
It could fill a major supply gap as the 18 million doses of RTS,S available for rollout in 2023-25 fall well short of the needs of malaria endemic countries, says the WHO.
"This second vaccine holds real potential to close the huge demand-and-supply gap," said Matshidiso Moeti, WHO director for the Africa region, where nearly half a million children die from the mosquito-borne disease each year.
African vaccine manufacturing
In 2021, the African Union launched the Partnerships for African Vaccine Manufacturing, an initiative coordinated by Africa CDC which aims to make 60 per cent of the vaccines needed in the region by 2040.
Africa CDC's Ndembi said that the technology needed to be transferred to the 30 manufacturers identified under the initiative "so we can manufacture them and make them available to our population".
The Matrix-M part of the vaccine name relates to patented technology developed by US biotechnology company Novavax to enhance immune response, known as an adjuvant.
SciDev.Net asked Novavax on Thursday (5 October) if it would be willing to share the intellectual property with manufacturers identified under the Partnerships for African Vaccine Manufacturing. However, SciDev.Net had not received an answer by the time of going to press on Friday (6 October).
The Serum Institute of India declined to comment on whether it would share intellectual property on R21 with African manufacturers. No response was received from the University of Oxford by the time of going to press.
Cost
The vaccine is expected to cost between US$2 and US$4 per dose - more than the daily budget for many of the world's poorest who need it most. The WHO says this is comparable with other childhood vaccines.
Over 400 million people in Sub-Saharan Africa live on less than $1.9 per day, according to UN data.
Dorothy Achu, team lead on tropical and vector-borne disease at the WHO African regional office, said malaria vaccines could be supplied as "vaccine grants" through UN agencies, so endemic countries would receive an allocated number of doses without having to pay for them.
She said children, including those in rural areas, would be immunised as part of a national immunisation programme from the age of five months, with a scheduled course of follow-up doses.
"So, wherever vaccination services exist, the children will be able to access the malaria vaccine," she said.
"The only role that countries need to play now is to order the vaccines on time and make sure that they are distributed to all vaccine sites, and that the health workers are trained on how to store and how to administer the vaccines to these children."
At least 28 African countries plan to introduce a WHO-recommended malaria vaccine as part of their national immunisation programmes and Gavi, the Vaccine Alliance has agreed to provide technical and financial support for rollout in 18 of these.
The RTS,S vaccine will be delivered in some countries in early 2024, while the R21 malaria vaccine is expected to become available in mid-2024, the WHO said. It stressed that both vaccines were equally safe and effective in preventing malaria in children.
100 million doses
The Serum Institute, which has the licence to the R21 vaccine, has established production capacity for 100 million doses a year. It says this will double over the next two years.
R21 received WHO recommendation following advice from the WHO's Strategic Advisory Group of Experts on Immunization (SAGE) and the Malaria Policy Advisory Group (MPAG) following a meeting last week (25-29 September).
The vaccine is now undergoing the process for WHO prequalification so that it can be rolled out globally where needed. Once it has this authorisation, it can be offered through Gavi's vaccine programmes, which individual countries need to apply for.
Peter Baker, deputy director of global health policy and policy fellow at the Center for Global Development, said the approval could be "game-changing" in the fight against malaria.
"However, this will depend on the finances of malaria-endemic countries and their donors, which, since the COVID-19 pandemic, has been challenging.
"Every dollar spent on R21 is a dollar not spent on potentially more cost-effective malaria prevention measures, such as bed nets."
He said future WHO guidance should "avoid a one-size-fits-all approach and instead support local decision-making" based on countries needs and budgets.
This piece was produced by SciDev.Net's Global desk.