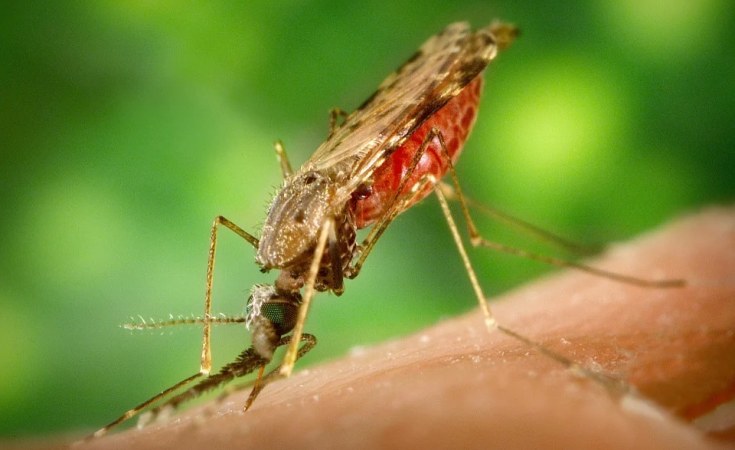
Children are at higher risk of the mosquito-borne disease which kills more than 600,000 people each year, according to WHO African Region.
Ghana has approved a new malaria vaccine from Oxford University, becoming the first country in the world to take that step forward in fighting the life-threatening disease.
Oxford, in collaboration with the Serum Institute of India, will produce up to 200 million doses of the vaccine - called R21/Matrix-M - annually with a vaccine factory being constructed in Accra, Ghana, the BBC reports.
While regulatory bodies, including the World Health Organisation (WHO), are still assessing the safety and effectiveness of the vaccine, Ghana's drug regulator has approved it domestically for children aged 5 months to three years old.
The effort is one of several focused on addressing the disease that kills over 600,000 each year, most of which are children. The complicated structure and lifecycle of the malaria parasite have long stymied efforts to develop vaccines.
Fighting malaria
The R21/Matrix-M vaccine is the second to ever be approved by the WHO and the first to exceed the WHO threshold of 75 per cent efficacy over 12 months of follow-up.
The vaccine showed a 77 per cent protective efficacy over 12 months in a phase 2b trial involving young West African children, following an initial three-dose course of injections.
The first-ever malaria vaccine, RTS,S or mosquirix, from British drugmaker GSK, was approved by the WHO in 2021 after decades of work. But a lack of funding and commercial potential thwarted the company's capacity to produce as many doses as were needed.
Various research also shows that the effectiveness of GSK's vaccine is approximately 60 per cent, and significantly wanes over time, even with a booster dose.
At the time mosquirix vaccine was approved, the WHO said it was based on results from an ongoing pilot programme in Ghana, Kenya and Malawi that has reached more than 800,000 children since 2019.
It added that it is recommending widespread use of the vaccine, "among children in sub-Saharan Africa and in other regions with moderate to high plasmodium falciparum malaria transmission.
Towards Malaria eradication
The BBC also reports that the Director of the Jenner Institute at the University of Oxford, Adrian Hill, stated that the R21 vaccine is expected to "make a major impact on malaria mortality in children in the coming years.
"And in the longer term, it will contribute to the overall final goal of malaria eradication and elimination."
Each dose of R21 is expected to cost a couple of dollars.
Also, the CEO of the Serum Institute, Adar Poonawalla said: "Developing a vaccine to greatly impact this huge disease burden has been extraordinarily difficult."
He added that Ghana, as the first country to approve the vaccine, represents a "significant milestone in our efforts to combat malaria around the world".